The uranium had been extracted by dissolution in sulfuric acid leaving radium sulfate which is similar to barium sulfate but even less soluble in the residues. Uranium is the heaviest of the naturally occurring elements.
Uranium was found to be radioactive in.

What is the history of the element uranium. Atomic bomb is also made using Uranium. He named uranium after the then newly discovered planet Uranus. The lustrous black powder that the chemist Klaproth isolated from the mineral pitchblende in 1789 - just eight years after Uranus was discovered - was in fact an oxide of uranium.
It can be found in row seven of the periodic table and is a member of the actinide groupUranium atoms have 92 electrons and 92 protons with six valence electrons. In the first extraction of radium Curie used the residues after extraction of uranium from pitchblende. In the United States mines popped up out West in the Colorado Plateau an area that unites the corners of Utah Colorado New Mexico and Arizona.
Rocks that contain a lot of uranium are called uranium ore or pitch-blende. The half-lives of its naturally occurring isotopes range between 159200 years and 45 billion years. Uranium has in the past been used to make yellow to green coloured glass that fluoresces green in ultraviolet light.
The use of uranium in its natural oxide form dates back to at least AD 79 when it was used to add a yellow color to ceramic glazes yellow glass with 1 uranium oxide was found near Naples Italy. They can be found in low concentrations almost everywhere in rock soil rivers and oceans. The uranium ore was only a byproduct of the mining activities.
You can find this element on the periodic table of elements by looking for the U symbol or atomic number 92. Uranium was discovered by Martin Heinrich Klaproth a German chemist in the mineral pitchblende primarily a mix of uranium oxides in 1789. It is widely used to fuel nuclear power plants and to make high density penetrators.
Uranium was discovered by Martin Heinrich Klaproth a German chemist in the mineral pitchblende primarily a mix of uranium oxides in 1789. Uranium Research at the NM Bureau of Geology. Uranium was formed over 66 billion years ago.
Uranium is a weakly radioactive element discovered in 1789 by Martin Heinrich Klaproth. Uranium although abundant is a nonrenewable energy source. Uranium is a chemical element with the symbol U and atomic number 92.
Natural uranium contains about 07 of the U 235 isotope the fissile isotope and 992of the U 238 isotope. It is the principle fuel for nuclear reactors but it also used in the manufacture of nuclear weapons. Uranium and thorium are common elements in the Earths crust.
Becquerel discovered that uranium exhibited invisible light or rays. These numbers refer to the number of. Due to its high density the element has found use in inertial guidance devices and in gyroscopic compasses.
He named uranium after the then newly discovered planet Uranus. Eugène-Melchior Péligot a French chemist isolated pure uranium in 1841 by heating uranium tetrachloride with potassium. Uranium was discovered in 1789 by the German chemist Martin Heinrich Klaproth.
Uranium was discovered by German Chemist named Martin Heinrich Klaproth in Berlin 1789. Uranium U is a radioactive element that averages one to four parts per million in the Earths crust. It was also used for tinting in early photography.
Humans have actually been aware of this substance for a very long time. Although Klaproth as well as the rest of the scientific community believed that the substance he extracted from pitchblende was pure uranium it was actually uranium dioxide UO2. Uranium was discovered in 1789 by Martin Klaproth a German chemist in the mineral called pitchblende.
See uses of depleted uranium. Uranium was apparently formed in supernovae about 66 billion years ago. Although Klaproth as well as the rest of the scientific community believed that the substance he extracted from pitchblende was pure uranium it was actually uranium dioxide UO 2.
Uranium was formed when the Earth was created and is found in rocks all over the world. Just like all matter the history of uranium and nuclear energy can be traced back to the atom. Martin Klaproth a German chemist first discovered uranium in 1789 by extracting it from a mineral called pitchblende.
Uranium Resources in NM. It is a silvery-grey metal in the actinide series of the periodic tableA uranium atom has 92 protons and 92 electrons of which 6 are valence electronsUranium is weakly radioactive because all isotopes of uranium are unstable. It is one of eight elements named in honour of celestial objects but you might not think that uranium deserves to be named after the planet Uranus.
It was named after the planet Uranus which had been discovered eight years earlier. In 1896 Antoine H. Physical properties of Uranium.
There were no major uses of uranium until 1934 when it was discovered that uranium could emit beta rays when inundated with neutrons. As early as the first century CE uranium oxide was used in glass and pottery colorants. From Discovery to Fission.
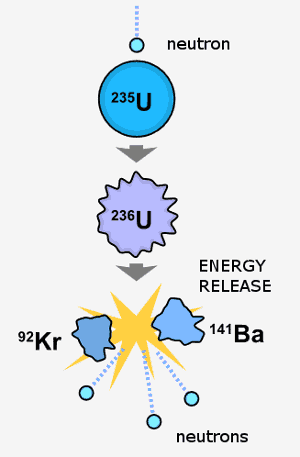
Uraniums debut as a nuclear element dates back to the top-secret Manhattan Project through which scientists developed the first atomic bomb. Uranium U is a metallic silver-gray element that is a member of the actinide series. The element uranium became the subject of intense study and broad interest after German chemists Otto Hahn and Fritz Strassmann discovered in late 1938 the phenomenon of nuclear fission in uranium bombarded by slow neutrons.
In 1934 research by Enrico Fermi and others eventually led to the use of uranium fission in the first nuclear weapon used in war and later in the peaceful use of uranium as fuel in nuclear power production. Martin Klaproth a German chemist first discovered uranium in 1789 by extracting it from a mineral called pitchblende. Just like all matter the history of uranium and nuclear energy can be traced back to the atom.
Three isotopes of uranium are found in nature uranium-234 uranium-235 and uranium-238. Natural Decay Series of Uranium. The residues also contained rather substantial amounts of barium sulfate which thus acted.
Concentrations of uranium minerals such as uraninite carnotite and brannerite can form commercial deposits. Though it is not common in the solar system its slow radioactive decay provides a major source of heat within the Earth responsible for continental and convection drift. Uranium occurs in seawater and can be recovered from the oceans.

Periodictableofelements Periodictable Uranium Teaching Chemistry Science Infographics Chemistry

Uranium Chemical Element Periodic Table Science Symbol Science Symbols Chemical Elements Periodic Table Periodic Table Of The Elements

Uranium Facts Symbol Discovery Properties Uses

What Is The Element Uranium Used For Periodic Table Of The Elements Atom Periodic Table

The Open Door Web Site Chemistry Visual Chemistry Uranium

The Ions In Uranium Are All Positive Charge Most Common Ions Are U 3 U 4 Uo 2 Uo 2 2 Positivity Most Common Art